CATEGORY
Viral Vaccines
Beroe LiVE.Ai™
AI-powered self-service platform for all your sourcing decision needs across 1,200+ categories like Viral Vaccines.
Market Data, Sourcing & Supplier Intelligence, and Price & Cost Benchmarking.
Schedule a DemoCategory Alerts
The European Commission has approved a purchase agreement amendment for Valneva's inactivated COVID-19 vaccine.
July 27, 2022WuXi Vaccines Received Ireland Health Products Regulatory Authority's First GMP Certificate of QC Potency Lab
July 21, 2022Vaccizone has chosen Exothera to handle the process development and GMP manufacturing of its SARSCoV2 vaccine, which is based on proprietary ASC technology.
July 13, 2022Become a Beroe LiVE.Ai™ Subscriber to receive proactive alerts on Viral Vaccines
Schedule a DemoViral Vaccines Market Monitoring Dashboard
Understand the correlation between costs, margins, and prices impacting your category on a real time basis on Beroe LiVE.Ai™
Schedule a DemoViral Vaccines Industry Benchmarks
Savings Achieved
(in %)
The average annual savings achieved in Viral Vaccines category is 2.50%
Payment Terms
(in days)
The industry average payment terms in Viral Vaccines category for the current quarter is 90.0 days
Compare your category performance against peers and industry benchmarks across 20+ parameters on Beroe LiVE.Ai™
Category Strategy and Flexibility
Engagement Model
Supply Assurance
Sourcing Process
Supplier Type
Pricing Model
Contract Length
SLAs/KPIs
Lead Time
Supplier Diversity
Targeted Savings
Risk Mitigation
Financial Risk
Sanctions
AMEs
Geopolitical Risk
Cost Optimization
Price per Unit Competitiveness
Specification Leanness
Minimum Order Quality
Payment Terms
Inventory Control
The World’s first Digital Market Analyst
Abi, the AI-powered digital assistant brings together data, insights, and intelligence for faster answers to sourcing questions
Abi is now supercharged with GPT4 AI engine. Enjoy the ease of ChatGPT, now on Abi

Use the Viral Vaccines market, supplier and price information for category strategy creation and Quaterly Business Reviews (QRBs)
Schedule a DemoViral Vaccines market report transcript
Viral Vaccines Global Market Outlook
-
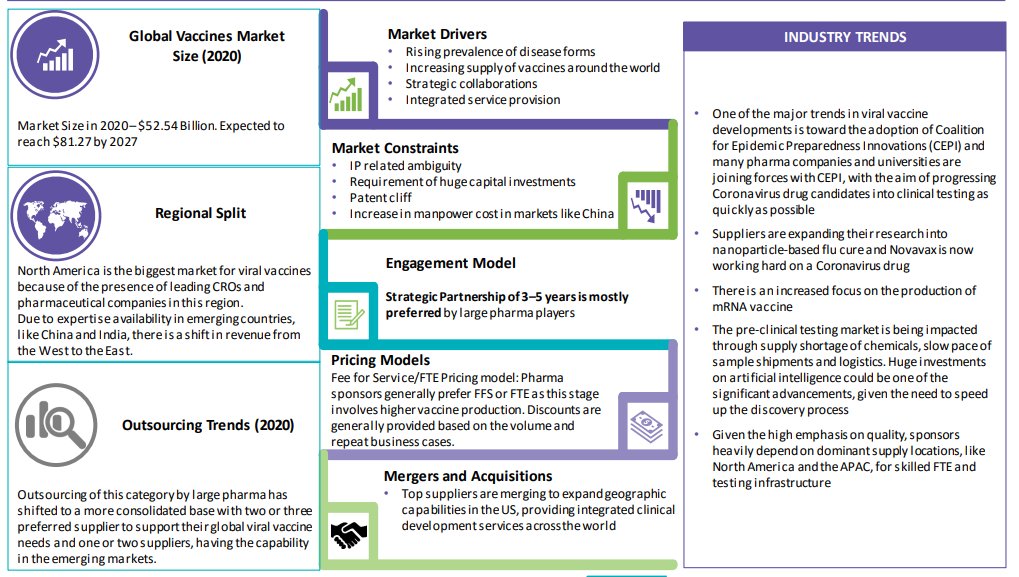
The global demand is expected to grow at approx. 7 percent CAGR through 2020–2027 and would reach approximately $ 58.4 Billion by the year 2024. 15-20% of the growth can be attributed to vaccine development and testing
-
Rise in prevalence of several infectious and non-infectious disease is promoting technological advancements in faster production of vaccines, and large number of international vaccination programs, government and non-government initiatives, and increase in demand for combined vaccine are responsible for boost in the market growth
-
Large CROs have been continuously expanding their supply base through acquisitions and partnerships to provide integrated services related to vaccine production
Global Market Size: Viral Vaccines
-
There is an increasing awareness of immunization and a inspiration to formulate strong vaccine pipeline and the increasing focus of the key pharmaceutical players to develop innovative vaccines. These factors are driving the market with a CAGR of 7 percent and is expected to reach $71.65 billion by the end of 2027.
-
The market dynamics of research chemistry can be understood from the demand–supply perspective
-
From the demand side, pharmaceutical companies are facing pipeline issues and outsourcing additional activities. This trend has led to the emergence of outsourcing of production services, with emerging nations like India and China becoming favorable destinations
-
The viral vaccine production function is highly outsourced to leverage technical expertise and specific therapeutic area skill set at CROs
Regional Market Share
-
It is easier to offshore more routine services, like Live-attenuated vaccine production and Subunit Vaccine production, to emerging regions, like India and China. However, services, like Toxiod Vaccines and Conjugated Vaccine Production that require strict regulatory guidelines, are mostly near shored to the developed markets
-
This is expected to change in the future with suppliers, like Wuxi Optec, building capabilities in technologies, like computer-aided drug development and identify potential drug candidates in lesser time. Such factors will expedite the drug development process
Cost Structure of Viral Vaccines
Cost for product development, equipment's and labor contributes a maximum of 65-70 percent of the overall agency base price, and comprises of direct cost (buying or manufacturing cost) and indirect cost (third-party engagement cost). Another factor is skilled labour cost, which differs widely, depending on the scope of the trials, location and the therapeutic area involved (e.g., average FTE cost depends upon the location and therapeutic area)
Price Drivers
-
The viral vaccines market is primarily driven by the rising prevalence of infectious diseases, innovative technology in vaccine development, increased funding from governmental and international organizations, and the rising awareness on preventive care.
-
This overhead cost component includes fees for service and facilities, technology deployment/up gradation cost, and out-of-pocket cost
-
Contribution to overall agency cost solely depends on the type of trial and location of the manufacturing
-
Labor cost includes direct payroll cost and the cost of temporary players or third-party engagements
-
Labor cost will continue to increase (at an average rate of 2-3 percent) as vaccine production requires specialist attention, and will drive extensive third-party resource allocation
Interesting Reads:
Discover the world of market intelligence and how it can elevate your business strategies.
Learn more about how market intelligence can enable informed decision-making, help identify growth opportunities, manage risks, and shape your business's strategic direction.