Connected devices enable cost savings in health care


Abstract
Connected devices are smart sensors having a number of applications across verticals in different industries such as healthcare, automotive, military and aviation, communications and others.
In today’s world, pharma and medtech companies are unable to monetize on a product’s success due to lack of key insights on real time data. Constant inflow of data from patients can help healthcare companies improvise and customize their products to drive the top line and also minimize the cost of therapy.
On account of advances in healthcare technology, market is filled with number of wearable devices, that can monitor various health parameters as a standalone device or a single wearable with multiple biosensors monitoring diverse parameters. Apart from monitoring vital signs these devices also collect, transfer and store real time data in cloud aiding health care professionals (HCPs) to predict and adjust the precise and accurate drug dosage levels.
In simple terms, wearable devices monitor patients’ diseases or medical conditions at their homes thereby avoiding or reducing frequent hospital visits. For example, Phillips IntelliVue Guardian wearable patch can be used to monitor patients at home and hospitals.
This paper outlines an overview with a case on how pharmaceutical and medical technology companies by using wearables (smart sensors) can minimize the spend incurred during the product development (Research and Development – clinical trials) stage.
Apart from minimizing the spend, companies can continuously monitor the product performance post the product launch and reduce the risk levels of product failures thereby resulting in better return on investments during the product lifecycle stage.
Overview of pharma research and development and healthcare connected devices in general.
Pharma research and development:
Healthcare research organizations predict that global medicine use in 2020 will reach 4.5 trillion doses, up by 24 percent on an average from 2015. This clearly indicates that the industry is constantly growing. However, it is also observed that close to half of this growth is driven by increased use of medicines by emerging nations such as India, China, Brazil and Indonesia.
It is also believed that by 2020 more than 90 percent of the prescription drugs in the U.S. are dispensed as generics.
According to the pharmaceutical industry, there are dozens of medical conditions currently which have no or very limited treatment solutions. It predicts that close to 230 medicines and around 75 new orphan drugs will be available in the markets by 2020. Of which more than one third caters to cancer therapy and rest caters to autoimmune disorders, heart disease, hepatitis C and an array of rare disease conditions.
As mentioned earlier, there are number of products (pharma and medical devices) that are in the research and development phase. They need to be monitored and evaluated for safety and efficiency.
Companies in pharma and med tech segment on average spend around 5 percent to 12 percent of their revenues on research and development (include clinical trials) in bringing efficient and safer products to the markets. It is understood that using connected devices in clinical settings can reduce the total cost of the trials and thereby reduce the overall product cost to an extent.
Healthcare connected devices:
Healthcare connected wearable devices are generally used for monitoring various vital signs parameters such as blood pressure, heart rate, respiratory rate, cough and sleep quality. Healthcare organizations across the globe are trying to use connected solutions to create more patient-centric services, while reducing the costs and simultaneously providing high-quality care and therapeutic solutions.
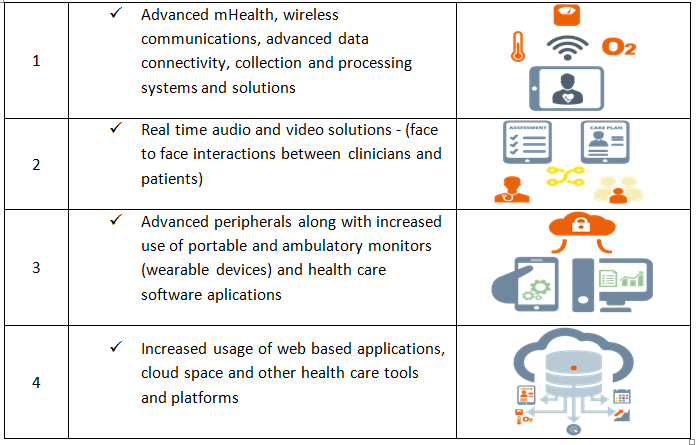
The following table indicates the key reasons for advances in usage of connected devices in healthcare ecosystem.
Key factors leading to connected healthcare industry advancement
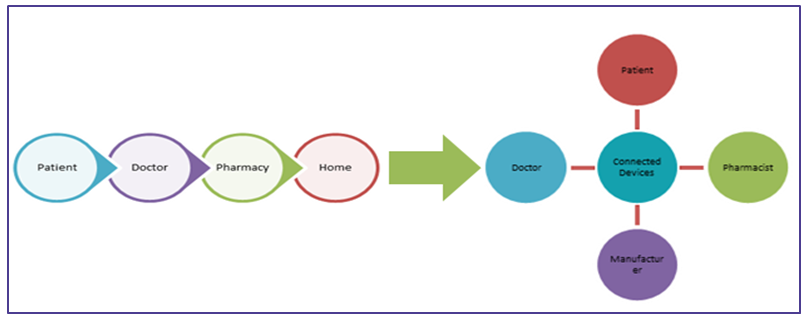
The above mentioned advancements have changed the traditional healthcare system to connect healthcare systems as given below in the image.
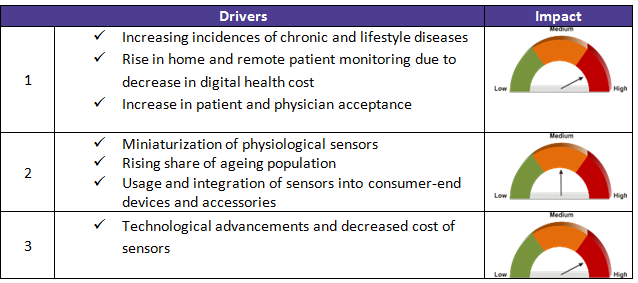
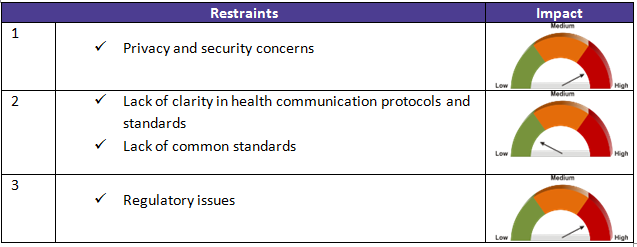
There are other factors (drivers and constraints) that also contribute to the advancements in health care connected solutions. These are described in detail in the table below along with their impact level (high, low, medium).
Market drivers and restraints: impact on healthcare ecosystem
Opportunity:
Implementation of connected technologies and devices such as wearables, mobile apps and other technologies from the clinical settings perspective can create potential cost saving opportunities for companies in healthcare.
Especially in quantitative assessment of disease conditions such as behavioral and mental disorders, nervous system diseases such as Parkinson’s disease, Alzheimer’s disease, cerebral infarction, seasonal affective disorder, restless leg syndrome, vascular dementia and also others such as sleep disorders related to abnormal activity or disorder symptoms.
The following case analysis helps to understand the usage of sleep monitoring devices on subjects in sleep disorder clinical studies and how it can help manufacturers to reduce the cost of studies as part of research and development spend.
Case analysis - benefits of using wearables on subjects in clinical settings for monitoring sleep quality.
Today’s market gives an opportunity for companies to use a single wearable that can monitor various parameters such as activity levels (steps and distance), sleep quality, blood glucose levels, heart rate and other key parameters used to understand the medical progress conditions in hospital and home set up.
Let us take an example of a sleep research study that needs to be done on 100 subjects for 50 days.
Let us assume that on average sleep laboratory charges around $500 each per day.
Total cost for this study accounts close to $2,500,000 ($500 per day*100 subjects*50 days).
Company using wearables to track the sleep quality can save more than 50 percent of the above mentioned spend which is more than $1,250,000 (2,500,000/2) per single study.
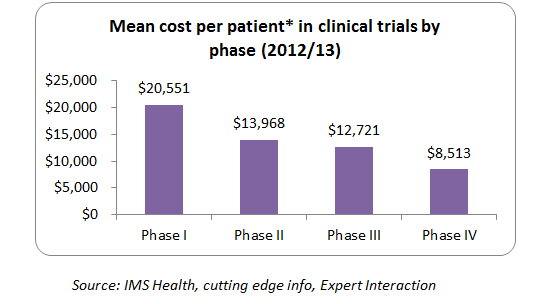
Also below graph indicates the high level average cost per patient at different trial phases in general spend by manufacturers during the research and development phase. (Note: cost varies across therapeutic areas, regions and compound type)
For example, let us assume that a company needs to do this kind of study for 5 times which costs about $12.5 million (5*2.5million). If companies use wearables, there would be a great scope of cost reduction (close to 50 percent on their spend) across different clinical trial phases such as phase II, Phase III and Phase IV respectively. So to sum up, on an average, companies implementing wearable solution in the clinical stings for some of the possible therapeutic studies can reduce the overall cost close to 50 percent of the spend across various clinical trial phases. (Note: cost varies across therapeutic areas, regions and compound type)
Apart from the above mentioned cost savings, other key benefits include:
- Increased patient retention and engagement opportunity on account of increased convenience for the patients
- Indirectly reduced need for number of on-site clinical staff (nurses, primary physicians, clinical coordinators and other support staff)
- Minimizing the number of patient care centers or partnered hospitals or clinical trial centers
Conclusion:
Implementing advanced connected technologies or solutions (wearables) in clinical setting can increase the research and development productivity. They can simultaneously minimize the supply chain issues and simplify decision-making process which leads to sustainable product development solutions across pharmaceutical and medical devices industry.
Related Insights:
View All
Get more stories like this
Subscirbe for more news,updates and insights from Beroe