Adopting demand led supply model offers multiple benefits to clinical research

Abstract
Clinical research has become globalized, patient-centric and complex over the past decade. Evolving demands of sponsors is creating a strategic imbalance in the supply chain of drugs. Traditional models of clinical trial supplies either work on supply-led or just-in-time models. They are more rigid and lack flexibility, speed and efficiency. This results in shortage or high wastage of supplies which can jeopardize the execution of trials and increase the cost of studies. Demand Led Supply (DLS) is a continuous GMP approach to clinical studies which decouples the primary and secondary packaging and empowers labelling and distribution of clinical supplies on a regional basis. Clinical research incorporating DLS reduces clinical waste to less than 20 percent (which is > 200 percent otherwise) and pool supplies across protocols.
Introduction
The pharmaceutical industry is now aiming at drug development for complex and expensive cell therapies and large molecules with main focus on rare diseases. In parallel, clinical research is globalized to access large populations of treatment-naïve patients, to reduce cost of the trials and to bring drugs to market as quickly as possible. There is a significant increase in the number of clinical sites to individual trials especially those involving temperature-sensitive drugs.
Globalization of clinical trials, shift towards large molecules, temperature-sensitive drugs, patient-centric approach, regulatory changes, and cultural expectations are challenging the clinical supply chain making it more complex in recent times. Clinical supply chain providers have started adopting new approaches to increase speed, flexibility and efficiency to ensure that medicines reach the patients participating in a trial at the right time and in the right dosage.
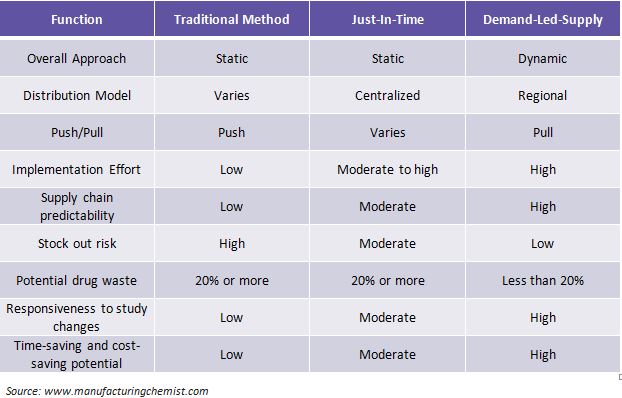
Traditional packaging and labeling involves bulk preparation of clinical supplies which require long lead times and has less flexibility. Wrong assumptions made in the supply chain results in CTM overages and wastes. This means that some kits are returned for over-labeling while the others are destroyed. To tackle these issues, there are better alternative solutions such as ‘On Demand’ packing, ‘Just-In-Time’ (JIT) Packing and ‘Demand Led Supply’ (DLS). Among these, the DLS model reduces burden on pharma by offering a high degree of flexibility with reduced timelines and increased speed in distribution from regional facilities.
Industry Shift - Demand Led Supply
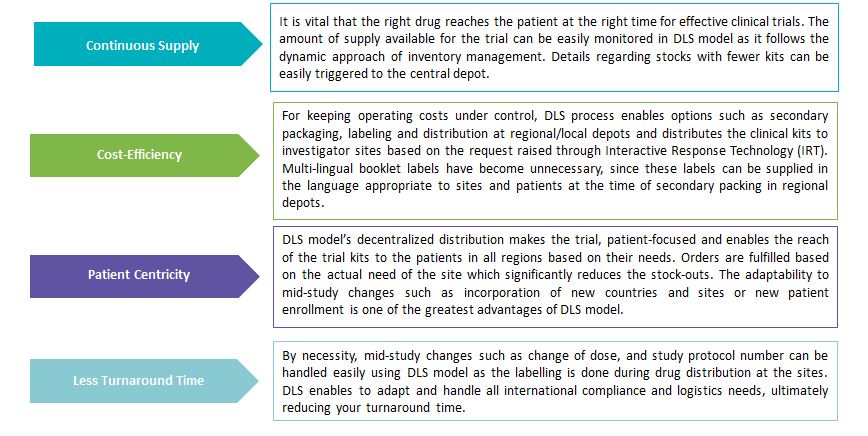
- Demand Led Supply model is a dynamic, continuous GMP secondary packaging, labeling and distribution process in which the primary packing of the medication is performed at a central location along with the labeling process, describing the basic information of the drug.
- The primary packed drug is then distributed to multiple regional facilities where the drug is stored until a request comes through IRT. The relevant country label with study-specific protocol and patient-specific information is printed on the previously created labels prior to shipping, once there is a new request. This helps the patients to know required information quickly and clearly.
- DLS produces “bright stock” with a batch-lot barcode and undergoes necessary analysis and quality testing. All the information is loaded into centralized inventory tracking system to record the supply movement and availability. The primary packed trial material is packed at regional depots with country-specific labels and distributed to the clinical sites.
Benefits of adopting DLS Model
Comparison of Traditional, JIT and DLS models
Challenges in implementing DLS
Regional differences: Since secondary packaging process is done regionally, different standard operating procedures, endpoints and regulatory methods are to be followed according to a particular region.
Investment and infrastructure: DLS model requires upfront investment, significant time, resources and expertise for developing decentralized GMP packaging, assembly and distribution. Printers, scanners, cameras and ancillary packaging equipment which follow GMP standards are required for secondary packaging.
Conclusion
Like any element of a trial, clinical trial supplies using Demand Led Supply model raise questions in many ways.
DLS model optimizes the clinical supply chain by reducing the cost, redundancy and complexity, increasing speed, flexibility and efficiency of trials. However, pharma can try a hybrid model that includes “Demand Led Supply” and “Direct to Patient” to ensure better results when compared to individual models.
Electronic supply practices are taking shape in the perpetual world of clinical trials where new technologies such as smart booklet labels with electronic displays that allow study pooling and expiry updates can optimize DLS model in the future.
Related Insights:
View All
Get more stories like this
Subscirbe for more news,updates and insights from Beroe