
The Advantages, Risks, and Future of Single-use Technology in Biopharmaceutical Production

Abstract
Introduction:
The global biopharmaceutical industry is growing substantially with strong and large pipelines, and the biologics market is forecasted to reach USD 450 billion by 2025. However, the biopharmaceutical companies still face ample challenges which are yet to be conquered. Such challenge includes the need to bring products to market at a faster rate and low cost in accordance with globalization, GMP requirements, and price pressure. Hence, the advent of single-use technology (SUT) has emerged as an important solution for biopharmaceutical companies to cash upon saving avenues and shorter time-to-market.
Single-use technology (SUT) refers to products or solutions, which are intended for a one-time use. Such objects are usually made from plastic (polyamide, polycarbonate, polyethylene, polyether sulfone, polypropylene, polytetrafl urethylane, polyvinyl chloride, cellulose acetate, ethylene vinyl acetate) and are disposed of after use. Such SUT enable biopharmaceutical manufacturers to move away from equipment that needs to be sterilized or consumables that are recycled or pose a risk with their transfer into clean-rooms. SUT has become a mature technology and many global pharmaceutical and biotech companies have garnered significant experience in both API manufacturing, upstream as well as downstream, as for the fill-finish part for drug substance or drug product.
Categorization of single-use products:
The single-use products can be categorized into systems for everyday lab use, simple peripheral systems and systems for basic operations and process platforms, as shown in Fig 1 below.
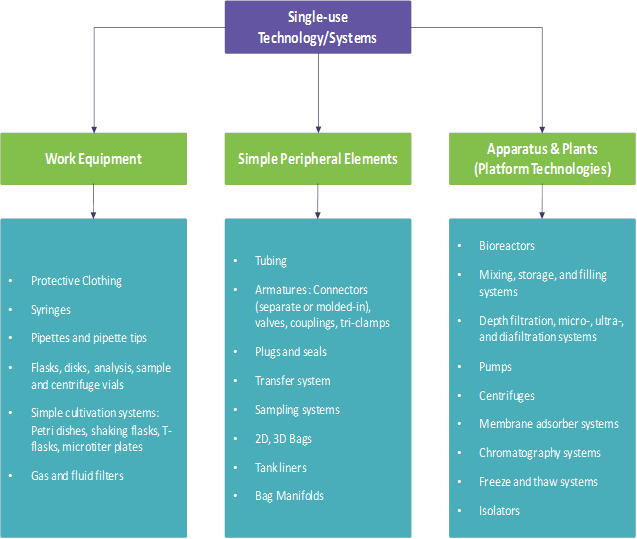
Fig 1: Categories of single-use products
Source: DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie e.V.
Advantages of single-use technology:
For any biopharmaceutical company, irrespective of the challenges pertaining to speed to market, flexibility, and product quality or operator safety, single-use technology acts as an optimal solution. SUT has many advantages, with reductions in investment and variable costs as the most commonly cited benefits; yet they represent only one aspect of a strategically important decision in production technology. Today, single-use technology presents a multitude of front-end considerations such as, process compatibility, leachables/extractables, film selection, back-up supplier and waste management. Along with this, the quality assurance system must be extended to cover both raw materials and production technology.
The major advantages for investing in single-use technologies are as below:
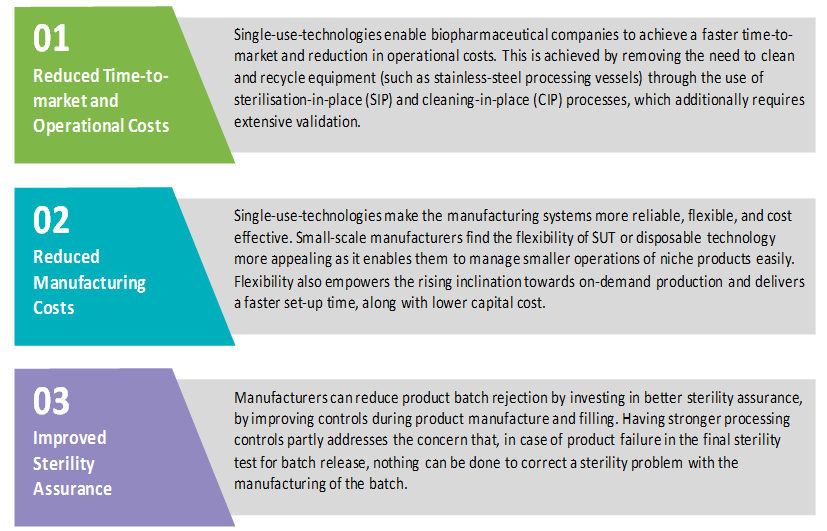
Hence, the advantages of single-use technology can be summarized as: eliminating cleaning needs; removal of in-house sterilization requirements (typically by autoclaving) for all components; reducing usage of cleaning chemicals; cutting storage requirements; lowering process downtime; and increasing process flexibility and reducing cross contamination risks.
Risks associated with single-use technology:
Although single-use technologies has brought in a lot of benefits for the biopharmaceuticals industry especially in terms of, flexibility, execution speed, investment cost, area need, reduced cleaning, validation, and ease of change-over. However, the reality of SUT is much more than manufacturing disposable plastic items. Single-use technologies have become a complex component of operations and require coordination of many factors to be successful.
The major risks associated with single-use technology include; film related risks, unit operation risks, operation risks, and supply chain risks which are listed below:
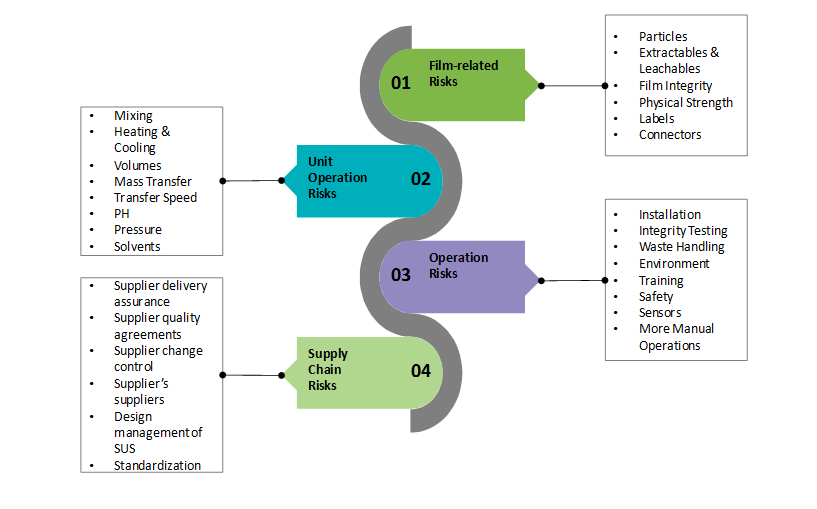
So, it becomes difficult for biopharmaceutical manufacturers to trust single-use technology implementation into bio-processing process steps and applications. The industry had witnessed evidences where, a single bag failure have cost manufacturers between USD 100,000 and USD 1 Million and that, each year, leaks in single-use systems have resulted in the loss of product valued at up to USD 20 million. So, changes to raw materials used in production of single-use systems needs to be analyzed and studied by manufacturers. This process itself is estimated to cost USD 100,000 per change.
How does single-use technology compete with the traditional stainless steel usage in biopharmaceutical manufacturing?
Single-use technology stands over stainless steel usage in terms of investment costs, as single-use systems require less initial costs for capex. It is because single-use systems require less instrumentation and fewer utilities. Also, SUT eliminates the sterilisation and cleaning processes along with a reduction of installation and support systems. This advantage benefits a manufacturer to purchase more capacity for a limited budget, but also has an effect on the variable costs because a much lower investment sum has to be amortized, compared with stainless steel. Hence, the low up-front investment cost can be credited to lower the variable cost which ultimately favours the use of single-use systems.
Comparison: Single-use VS Stainless-steel
Single-use systems can be stacked or moved in certain volume ranges as they have lesser utility requirements. Also, because of their improved designs and mobility along with a decreased demand for instrumentation, piping, valves, and maintenance; they occupy lesser space as compared to fixed systems. However, they still require space for transport and waste removal, manipulation etc. which still stands at lesser footprint than traditional fixed systems such as stainless steel.
|
Sr. No. |
Item |
Single-use (SU) |
Stainless-steel (SS) |
|
01 |
Investment costs |
Low |
High |
|
02 |
Technical complexity |
Low |
High |
|
03 |
Maintenance costs |
Low |
High |
|
04 |
Production scale |
Limited |
Not limited |
|
05 |
Consumables demand |
High |
Medium |
|
06 |
Material supply |
High |
Medium |
|
07 |
Cleaning efforts |
None |
High |
|
08 |
Sterilization time |
None |
High |
|
09 |
Waste production |
High |
Medium |
|
10 |
Energy consumption |
Medium |
High |
|
11 |
Inventory and storage space |
Medium |
High |
|
12 |
Biological contamination risk |
Low |
Medium |
|
13 |
Chemical contamination risk |
Medium |
Low |
|
14 |
Product changeover |
Quick |
Slow |
|
15 |
Overall processing time |
Quick |
Medium |
Table 1: Major advantages and drawbacks of single-use equipment for bio-manufacturing
Source: Wissenschaft und Technik Biotechnologie
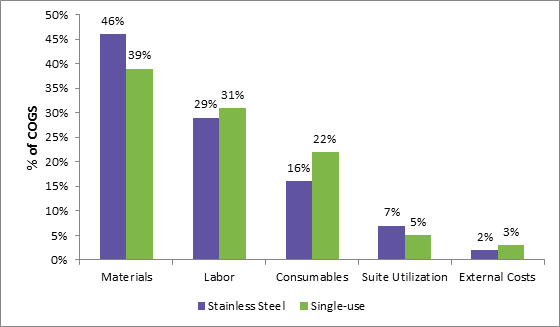
Fig 2: Cost comparison for biopharmaceutical manufacturing using stainless steel and single-use equipment
Source: Wissenschaft und Technik Biotechnologie
Future Trends in Supply Market:
The market trend leans toward increasing adoption of single-use equipment globally. Single-use equipment, especially for upstream manufacture (ex. Bioreactors) currently dominates the pre- commercial, i.e., small- to mid-scale R&D and trials supply manufacture, while fixed stainless steel equipment continues to dominate commercial-scale manufacturing. The industry anticipates an exceptional revenue growth as these systems enters into commercial manufacturing. Also, with more exploration of using hybrid systems (i.e. a combination of single-use and stainless steel technology) the industry is hopeful to witness innovations in the future.
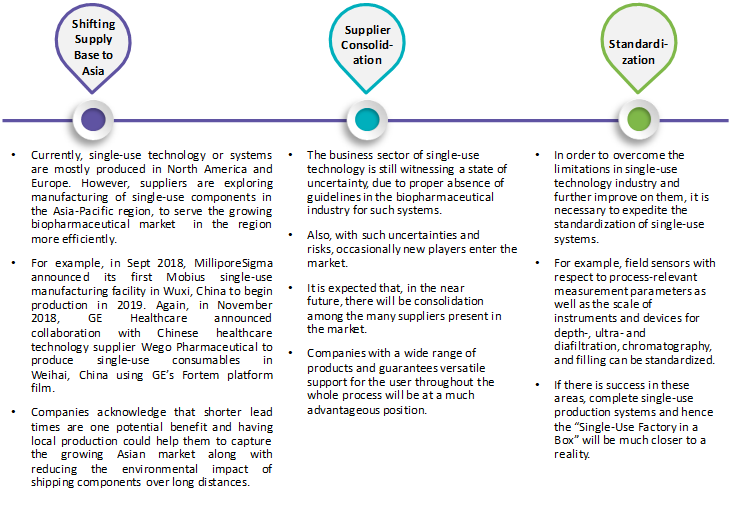
Future of single-use technology: Hybrid Systems
Currently, whether to use stainless steel or single-use technology is not the important issue. However, it is very necessary to identify a combination solution to use both these technologies in the most productive and cost-effective process in a fast and predictable way. Choosing a single technology or a combination of both depends on strategic considerations and feasibility studies as per every biopharmaceutical requirement. So, it is expected that the future will witness a rising use of hybrid systems and complete single-use systems for biopharma manufacturing facilities.
In biopharmaceutical manufacturing, a hybrid system is defined as the one which utilizes both fixed, reusable equipment (e.g. Stainless steel bioreactors) and flexible, single-use equipment. It is a system that comprises of both fully reusable and fully single-use equipment or systems. Example of such hybrid system is; using smaller single-use bioreactors at the start of a seed train while using stainless-steel bioreactors in the larger volumes of the seed train.
Conclusion:
Single-use technology has gained great significance all over the world for biopharmaceutical production. However, a major weakness of SUT, that affects all process levels and the single-use systems needed for them, is the lack of standardization and comparability among systems as well as with traditional systems. It is because; there has been no design and use recommendation for single-use systems or single-use facilities. Also, industry has neither witnessed any tests to assess the integrity of single-use systems prior to implementation, nor are there any regulatory requirements for single-use systems for the complete biotechnology process. Also, there are no evaluated analytical methods with acceptance criteria for leachables and extractables. So, it is anticipated that in the near future, SUT will be used beyond the biopharmaceutical field (food, cosmetics, etc.) and animal cell culture (microorganisms, plant cell and tissue cultures, algae.
References
- https://www.europeanpharmaceuticalreview.com/article/73939/strategy-for-the-adoption-of-single-use-technology/
- www.pda.org
- www.biopharma-reporter.com
- www.cpcworldwide.com
- Wissenschaft und Technik Biotechnologie
- www.dechema.de
Recommended Reads:
Related Insights:
View All
Get more stories like this
Subscirbe for more news,updates and insights from Beroe






