Successful patient recruitment calls for a calculated approach

Abstract
The competition among sponsors and CROs has been intensifying as the drug development processes become more complex and the regulations become more stringent. To curb the rising costs of clinical trials, sponsors are outsourcing almost all non-core activities while retaining R&D in-house. However, patient recruitment and retention are the greatest challenges faced by the clinical trial industry today. Both CROs and sponsors are plagued by the extended study timelines due to non-participation of study subjects on time. In addition to this, patient dropout rate across all clinical trials is around 30 percent and this needs to be analyzed to prevent study delays.
While the industry has been struggling from 2010 onwards to identify innovative means to engage patients, a majority of sponsors and CROs even today rely mostly on traditional tactics and are yet to adopt non-traditional methods. Though use of digital and social media is gaining traction, there is a lot of uncertainty around its usage and its potential to bring about the difference the industry has been waiting for. This whitepaper intends to highlight the current prevalent trends in engaging patients and will throw light on several innovative ways to recruit and retain patients.
Introduction
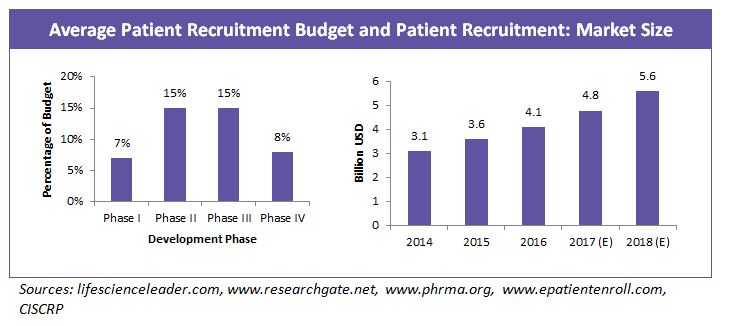
More than $2 billion is spent every year on recruiting patients for clinical trials, and the cost to start up a site is between $20,000-$30,000. On an average 60 percent of the drug development cost goes into clinical trials and 32 percent of the clinical trial cost goes into patient recruitment and retention.
Patient recruitment market is growing at a rate of about 16 percent annually as a result of increasing study complexities, regulatory requirements and competition in the industry to retain patients for successful study completion.
As per latest statistics, 85 percent of clinical trials fail to retain enough patients and almost 69 percent of the non-participation in trials is due to lack of awareness among the patients regarding the ongoing trials. Also people fear being treated like “guinea pigs” or being “experimented upon,” as well as not receiving treatment for their diseases.
In general, people lack faith in the medical profession based on past negative experiences. Other factors that influence patients’ decision to accept or decline participation in clinical trials include time constraints, distance from study site, transportation issues, and interference with work or home responsibilities.
Companies can address the perceptions of patients through continued and increased industry education. With effective communication they can have better study sites and have patients remain with a study until it is completed, thus saving time and money in clinical trials.
Pharma and CROs have been implementing innovative means to create awareness and attract patients.
Patient recruitment tactics
Recruitment of subjects remains the key focus in all clinical trials. The recruitment is based on the demographics of the target population and the conditions under investigation.
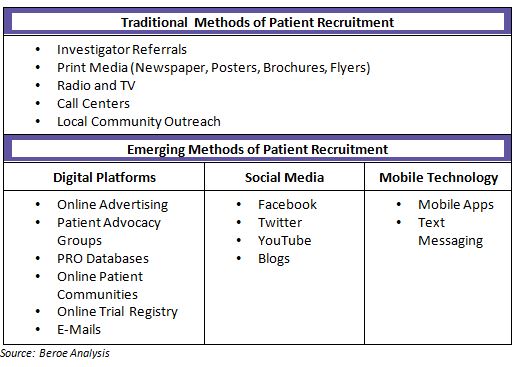
Patients can be reached through direct advertising by sponsors or through an advertising agency. Newspapers, radio and television are usually the platforms used to reach out to patients for direct campaigning. Additionally, potential participants can also be identified through clinical investigator’s practice site. The investigator or other supportive staff interact with the patients with medical histories or any new diagnosis for which they could be the potential participants for phase I-IV studies.)
Digital and Social Media
Majority of patients learn about clinical trials through online sources compared to traditional print or TV/radio outreach. Thus varied e-recruitment tactics are emerging like social media, online screeners, as well as web listening. In addition, with the growing popularity of mobile devices, use of text messaging and smart phone applications has become more widespread. In North America, about 11 percent of the trials involve social media communications.
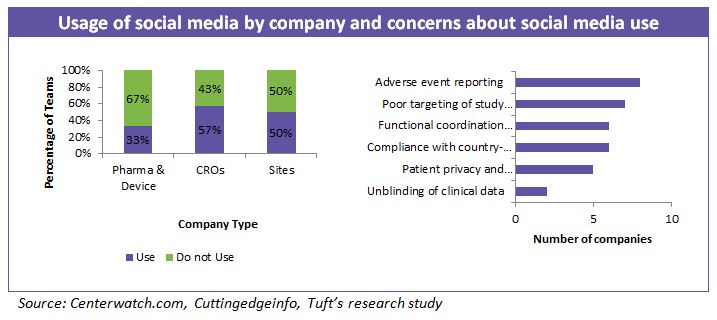
Majority of drug sponsors are primarily using social media for commercial purposes to distribute information (e.g., about drugs, diseases, and the company) and to listen to patient and professional conversations about marketed products, rather than for supporting clinical research.
Very few companies have reported use of social and digital media for patient engagement recruitment and retention. Of the 50 largest drug makers worldwide, only half are involved in social media, and just 10 use all three of the largest social media sites — Facebook, Twitter and YouTube, according to a recent report.
Companies are also reserved about exploring social media as there is a possibility that patients on a study may communicate treatment effects or side effects. This will essentially unblind a treatment and invalidate the results of a clinical study to a great extent.
Mobile applications are also used for reaching the patient community. 75 percent of biopharmaceutical companies and CROs have reported the use of mobile applications. Companies employ mobile strategies to enhance the clinical trial experience for patients. For example, making the participation easier and more convenient, increasing the likelihood of volunteers completing the study.
Mobile technologies are used mainly to promote study opportunities, prescreen potential patients, remind participants about their study obligations and collect clinical data from volunteers remotely.
Apart from other methods of recruitment, a minority of patients are also recruited from patient networks and advocacy groups. Overall usage of these types of organizations is fairly uncommon with current adoption level of 18 percent, but will continue to grow in coming years. As patients have become smarter they are increasingly utilizing resources such as the Internet and are using patient communities for discussions around their health and clinical trial experience.
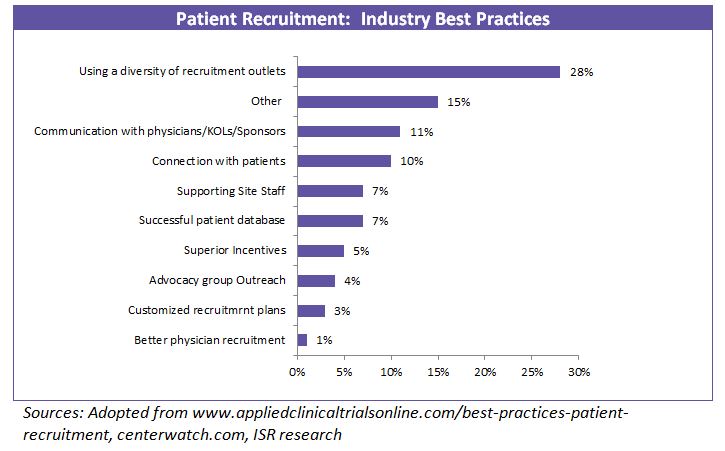
Since there are multiple methods towards achieving a successful recruitment strategy, companies have been utilizing a mix of strategies to gain efficiency in clinical trials.
Patient retention
The regulatory challenges of showing greater outcome data on new drugs in real-world patient settings have compelled sponsors, CROs, and patient recruitment agencies to explore more creative methods in patient outreach and retention, as well as better approaches in training and engaging science-focused sites and investigators on a recruitment campaign from the beginning.
Patient Recruitment Emerging Strategies
Retaining patients throughout the study completion is important to obtain final evaluative data. Patients cannot be retained without an initial pool of enrolled volunteers. This initial pool of screened participants depends on designing a successful patient recruitment strategy.
In line with this, most of the pharma companies and CROs are developing internal capabilities to enhance patient recruitment.
Some of the initiatives are:
Crowdsourcing of protocols: Generally conducted through face-to-face focus group meetings or online surveys, trial sponsors are able to obtain direct feedback from potential study subjects about overall study design and the requirements for participation. Incorporating this level of detail into the protocol design has the ability to positively affect enrollment and attrition rates, in turn closing the gap from clinical research to consumer market availability.
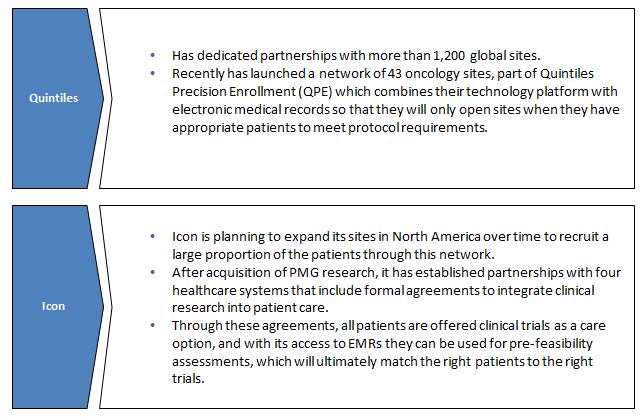
Networking with sites: The investigative sites have the ability to proactively identify, enroll, and develop a connection with study subjects which ultimately impacts the overall success of the program. As a result, majority of CROs have established relationships with healthcare networks, hospitals or universities that allow them to access patient data. This helps in understanding protocol design and patient recruitment feasibility. CROs have also developed several programs to better align themselves with sites, improve communication with investigators and address site needs.
Big data and analytics: Leveraging the data effectively helps companies to have an edge over others. Data provides a better understanding of where patients are located geographically down to a county or zip code. It can also be used to determine which physician and investigator is treating those patients, and whether the patients are on certain prescription-based medications or have undergone certain diagnostic testing. Data can also be used to provide personalized healthcare. In the future, its usage will be a necessity to drive the development of new treatments and significantly decrease clinical cycle times.
Leveraging technology platforms: Companies are either developing their own tools or partnering with suitable providers of innovative tools and technologies. It enables them to stay at the forefront of innovation. Technology helps to identify, engage, and interact with the individuals who wish to participate in research. It provides powerful and highly targeted mechanisms for patient identification, including the use of sophisticated insurance claims and electronic medical record databases, study specific websites with highly targeted search engine optimization and pay-per click advertising mechanisms, smart phone applications, text message appointment reminders, and many more.
Effective communication and building trust: Heath care providers, clinical trial sponsors, the media, and the public must maintain open communication to overcome real and perceived barriers to clinical research participation. Patient engagement involves all interactions between sponsors or sites and patients. These interactions occur during study planning, during data mining and feasibility activities, and throughout awareness and recruitment campaigns, screening processes, study conduct, and study follow-up and closeout activities. Further, sponsors have been experimenting with social media to educate patients and create awareness and to build relationships with advocacy groups.
The NIH is working to make the purpose, risks, benefits, and time requirements of trials more transparent. The Clinical Trials Transformation Initiative (CTTI), a public-private consortium co-founded by the FDA and Duke University, is preparing to issue new recommendations to researchers. It is urging them to work more closely with all stakeholders involved in the clinical trial process, including patients and advocacy groups. CTTI recommends, at a minimum, that patients be asked what questions are important to them, how many procedures they are willing to tolerate, and how written consent forms can be improved.
Conclusion
More than 60 percent of clinical trial participants get informed about the trials through web-based sources. Further digital tools and technologies help streamline clinical trial patient recruitment and registration. However, there is no one-size-fits-all strategy, and a combination of study branding and a messaging strategy based on findings from the patient-specific research results is recommended.
Success of clinical research trials from initiation to study close-out depends on how carefully study has been designed, and how efficiently the recruitment and enrollment strategies are being implemented. Additionally, higher retention rates throughout the engagement life cycle can be achieved through effective patient and investigator site education utilizing patient-centric media which includes paper, mobile devices, and websites.
Related Insights:
View All
Get more stories like this
Subscirbe for more news,updates and insights from Beroe